Wayne State University will host a clinical trial to rigorously test for one year the efficacy of a non-pharmacological medical device for Opioid Use Disorder, or OUD, that could become the first treatment of its kind for the disease.
“NET Device as a Non-Pharmacological Alternative to Medication for Promoting Opioid Abstinence,” is a U.S. Food & Drug Administration-qualifying clinical trial led by principal investigator Mark Greenwald, Ph.D., professor and Gertrude Levin Endowed Chair in Addiction and Pain Biology in the WSU Department of Psychiatry and Behavioral Neurosciences.

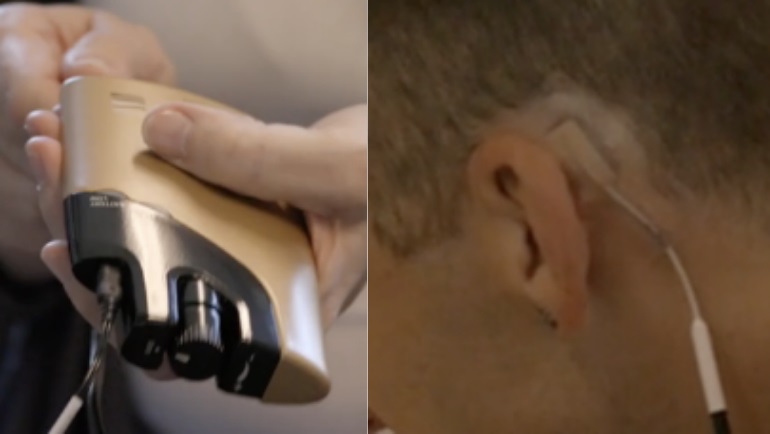
The NET, or NeuroElectric Therapy, device delivers alternating current via surface electrodes placed trans-cranially on the mastoid processes, located at the base of the skull on each side of the head. The electrodes are connected by a cable to a control box. The device produces low-amperage waveforms at proprietary controlled frequencies and pulse widths. The individual controls the intensity of stimulation by turning a knob on the control box.
NET has been used clinically for many years in Europe and South Africa, and pilot study data in Scotland and the United States has suggested it can help patients with OUD, Dr. Greenwald said.
FDA-approved Opioid Use Disorder medications are effective for many, but not all patients. There is a major unmet need for non-pharmacological OUD treatment alternatives for people who wish to be opioid-abstinent without medication, he said.
“Presently, OUD treatment engagement levels are unacceptably low – only about 15% of persons with OUD in the U.S. are in treatment. Many individuals want effective non-medication treatment options – for instance, think of participants in 12-step programs, which generally eschew medications – but these are presently limited. This could lead many OUD patients to not seek treatment or to drop out of treatment. So, we need to ‘meet patients where they are’ to get them into treatment and stay in treatment,” he added.
The FDA has stated the importance of expanding treatment options, especially non-opioid approaches that have lower abuse potential. Novel treatments for OUD are not intended to replace existing approved treatments, but instead to provide safe and effective alternatives to patients and clinicians. The results will be presented to the U.S. Food and Drug Administration for a clearance decision as soon as they’re available, hopefully Fall 2022, Dr. Greenwald said.
“Our initial work launched in early 2019 focused on analyzing the benefits of NET for opioid detoxification. We found that its efficacy for reducing opioid withdrawal and craving is comparable to FDA-approved medications. However, because opioid use disorder is a chronic relapsing disorder, we decided that focusing on the short-term benefit of detoxification might not be enough,” Dr. Greenwald said. “We decided to tackle the more challenging issue of seeing whether NET versus placebo stimulation during the inpatient detoxification period could have longer-term efficacy on outpatient opioid abstinence. If that’s the case, that would make a big impact, and would lead to the first-of-its-kind treatment in this disease space.”
Dr. Greenwald co-designed the clinical trial with sponsor NET Recovery Corp. Chief Executive Officer Joe Winston. Biostatistician and Associate Professor of Family Medicine and Public Health Sciences Samiran Ghosh, Ph.D., helped develop the statistical plan, will randomize participants to active NET versus placebo, with 50 patients per group (for 100 total), and will perform the statistical analysis.
All data collected for the trial – from Isaiah House Treatment Center, a clinical treatment facility in Willisburg, Ky. – will be sent securely to an electronic data capture system at WSU.
Dr. Ghosh and an associate will be the only “unblinded” individuals. The rest of the research team will not know which patients are receiving active versus placebo treatment.
“This is an unusually rigorous quadruple-blinded study,” Dr. Greenwald said. “We will not only measure outpatient abstinence from illicit opioid use, but will also evaluate whether NET can produce such abstinence without the need for FDA-approved medications. Secondarily, we are examining whether NET device use reduces non-opioid drug use. Taken together, these study features hold the device to a high standard. If successful, the device could either be used alone – and we would know its independent efficacy – or in combination with other approved treatments.”