
Researchers at the Wayne State University School of Medicine have discovered that a metabolite called itaconate produced during cellular metabolism plays a role in protecting the eye from abnormal inflammation during infection. The metabolite can be used with antibiotics to treat eye infections.
A metabolite is a substance formed in, or as an end result of, metabolism.
The study was led by Ashok Kumar, Ph.D., an associate professor in the Department of Ophthalmology, Visual and Anatomical Sciences and of the Kresge Eye Institute.
The findings, “Integrative metabolomics and transcriptomics identifies itaconate as an adjunct therapy to treat ocular bacterial infection,” are published in the May issue of Cell Reports Medicine, a journal by Cell Press.
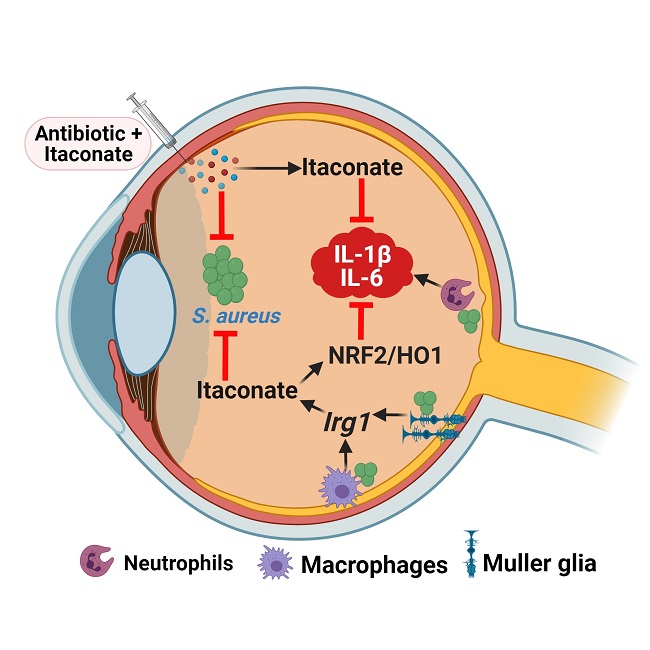
Itaconate is naturally produced by host innate immune cells to counteract inflammation and is non-immunosuppressive, making it an ideal adjunct therapy to control excessive inflammation in the eye.
“This therapeutic strategy is likely to improve visual outcome in bacterial endophthalmitis patients, where antibiotics will kill bacteria and itaconate reduces excessive inflammation. As some recent studies have shown the potential use of itaconate to downregulate inflammation in COVID-19, further research is under way in our laboratory to determine effects of itaconate on SARS-CoV-2 and other RNA viruses, such Zika, Dengue and Chikungunya,” he said.
His laboratory investigates how various bugs such as bacteria, viruses and fungi cause eye infections, and works to discover new therapies to prevent or treat them.
“Eyes are incredibly resistant to infection, however, ocular surgeries such as cataract or vitrectomy often predispose individuals to develop infection. As the aging population increases worldwide, there is increased demand for eye surgeries, hence, proportional higher incidence of acquiring postsurgical infections,” Dr. Kumar said.
Bacterial endophthalmitis is one of the most common infections after eye surgeries, when bacteria from the exterior of the eye such as the eyelid, conjunctiva or other surfaces gain access to the inside the eye. “The first event that happens inside the eye upon bacterial entry is the induction of inflammation, the body’s natural defense system to fight against infection,” he added.
Patients with bacterial endophthalmitis often experience pain, redness and blurred vision due to the mobilization of an arsenal of cells and molecules in the eye. Although the response is essential to contain infection, it often gets derailed and begins damaging tissues inside the eye, culminating in partial or complete vision loss. The current management of bacterial endophthalmitis involves local or systemic antibiotic therapy, which is highly effective in killing the bugs but does little to reduce inflammation. Ideal treatment should aim for both.
The researchers utilized animal and cell culture-based models to study the pathobiology of endophthalmitis caused by a variety of bacterial and fungal pathogens.
“In this study we decided to use high-throughput techniques of transcriptomics and metabolomics to visualize global changes in genes and metabolites during infection in the eye. These newer technologies are increasingly being used to uncover disease mechanisms and biomarkers, and we have utilized them in the past,” said Sukhvinder Singh, Ph.D., a postdoctoral fellow in Dr. Kumar’s lab and the study’s first author. “When we integrated our metabolomics and transcriptomic data, it provided us with the information on key gene and metabolic pathways altered in retinal tissue of bacterial-infected mouse eyes. Among various metabolites and genes, we observed a strong correlation between the expression of a gene named immunoresponsive gene 1 (Irg-1) and the production of a Kreb’s cycle metabolite called itaconate. Because itaconate was shown to be produced by immune cells, we sought to investigate its role in the eye, which was not known.”
The observation was made in the animal model, but the researchers questioned whether the changes also happen in human endophthalmitis. To find an answer, Dr. Kumar reached out to his longtime collaborator, Joveeta Joseph, Ph.D., a senior scientist and microbiologist at LV Prasad Eye Institute in Hyderabad, India. Dr. Joseph has a large biobank of vitreous samples from endophthalmitis patients.
“When they were assessed, surprisingly, itaconate levels were found to be elevated in vitreous of endophthalmitis eyes. This observation further supported our discovery in the mouse model and its translatability to human eye disease,” Dr. Kumar said.
They are also reviewing its applicability in the inflammatory eye disease uveitis, now treated with steroids. The immunosuppressive nature of steroids could cause harm during microbial infections, he said.
The study was supported by National Institutes of Health grants R01EY026964, EY027381 and R21AI140033.