A national study of the most common treatment options given to patients who experience refractory status epilepticus in the emergency department are equally safe and effective in children and adults.
The study encompassed three medicines -- Levetiracetam, Fosphenytoin and Valproic acid – administered to patients enrolled at 57 emergency department hospitals in the United States.
Associate Professor of Emergency Medicine Vijaya Arun Kumar, M.D., M.P.H., was the principal investigator of the study for Wayne State University, which encompassed Detroit Receiving Hospital, Sinai Grace Hospital and Children’s Hospital of Michigan in Detroit as study sites.
The results were published in “Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus,” included in a past issue of the New England Journal of Medicine.
The study was conducted by the Department of Emergency Medicine, Division of Clinical Research. Team members included Emergency Medicine Research faculty, physicians and research associates.
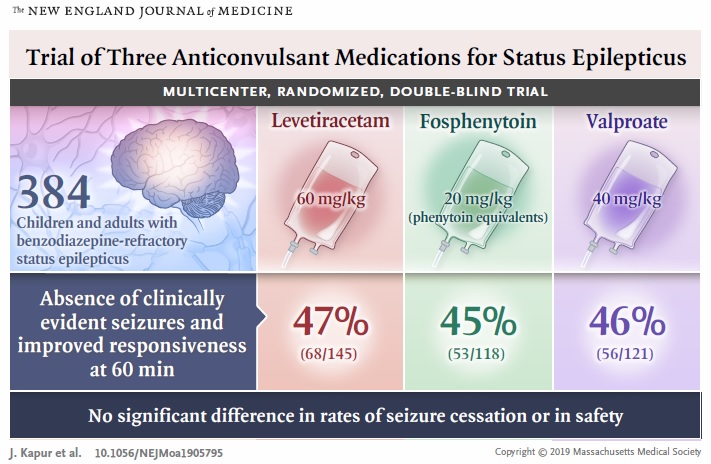
Nationwide, 384 patients were enrolled during a four-year period, and randomly assigned to receive one of the three drugs. “The results validated the use of either of the three drugs when it comes to treating patients with refractory status epilepticus,” Dr. Kumar said.
The researchers compared the efficacy and safety of the three intravenous anticonvulsive agents in children and adults with convulsive status epilepticus who were unresponsive to treatment with benzodiazepines. The primary outcome was absence of clinically evident seizures and improvement in the level of consciousness by 60 minutes after the start of drug infusion, without additional anticonvulsant medication. The posterior probabilities that each drug was the most or least effective were calculated. Safety outcomes included life-threatening hypotension or cardiac arrhythmia, endotracheal intubation, seizure recurrence and death.
The study was funded by the National Institute of Neurological Disorders and Stroke; ESETT ClinicalTrials.gov number NCT01960075.