Drs. Tongqing Zhou and Barna Dey have played a major role in the recent AIDS discovery made at the Vaccines Research Center at the National Institute of Allergy and Infectious Diseases that was featured in the February 15 issue of Nature. Dr. Dey received her Ph.D. from the Department of Biochemistry and Molecular Biology at Wayne State University's School of Medicine, and Dr. Zhou was a postdoctoral associate with Dr. Barry Rosen, chair of the department. Dr. Zhou also received his master's degree in computer engineering from Wayne State's College of Engineering.
Under the leadership of Dr. Peter Kwong from NIAID, the studies lead author, Dr. Zhou, Dr. Dey and other researchers at NIAID have discovered and mapped a small piece of HIV's outer coat that could be critical in developing a vaccine against this currently incurable disease. They have pinpointed a place on the outside of the human immunodeficiency virus that may be vulnerable to antibodies that could block it from infecting human cells.
This molecular target, according to the research team, which is a portion of the virus' gp120 surface protein, does not appear to mutate between strains. It also binds to an antibody that's already found in some humans. Finding a vaccine for HIV has been difficult because the organism mutates so much, constantly changing its outer sugary coating to elude detection. According to Dr. Kwong, "The trick has been to find a spot that stays the same between different strains and can also be accessed by antibodies that humans could produce in large numbers."
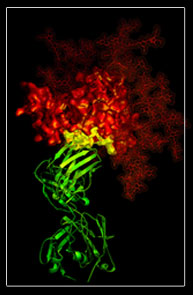
Making atomic-level images of the virus, this team of researchers revealed the structure of the protein, gp120, on the surface of HIV as it looks while the protein is bound to an infection-fighting antibody. This protein seems susceptible to attack by this antibody, b12, and is capable of broadly neutralizing the virus.
The research group found that while the virus uses the protein gp120 to gain entry into the CD4 T-cells it infects, the antibody b12 can block this process. Because the b12/gp120 connection does not change its configuration regardless of the HIV strain it is found on, these AIDS researchers feel it is the type of non-mutating "site of vulnerability" that vaccines may target someday.
These new findings are hopeful in helping to find a vaccine to curb AIDS. The next step is to do important animal research to see if the antibodies can be produced in animals, which will make it quite straightforward in human testing. The process is long, but the outlook is hopeful and this is a major step forward.
"It's exciting to think that what I'm doing at the lab bench today may help everyone in the future," says Dr. Zhou. "Just imagine a world without AIDS!"
"We are proud of the accomplishments of our graduates and the contributions that they are making to understanding the structural basis of diseases like AIDS," said Dr. Rosen.
For further information, see the full paper in the Feb. 15 issue of Nature.
http://www.nature.com/nature/journal/v445/n7129/index.html#rhighlts.